
Knappe also talked about his key takeaways from the panel discussion, which we’ll share further down in this story, under the subhead “Key Takeaways from the BMP Panel.” “I felt like most of the panelists were truly making an effort to be unbiased and data-oriented and that plays to our strength, so I was happy to see that.” “It was interesting to see the progression of thinking over time laid out as more and more data comes to bear on the question and it was done in a very academic way, but it was good to see it,” Brett Knappe, VP and general manager of Medtronic’s biologics business, told MD+DI in an interview at NASS soon after the panel session.
INFUSE BONE TRIAL
The timing of Wednesday’s panel session, “The Biologic Hasn’t Changed but the Evidence Has,” was interesting given Medtronic was already gearing up to announce on Thursday that FDA has signed off on a new Infuse trial that could lead to the product being cleared for a new indication. A panel at the North American Spine Society (NASS) meeting in Chicago, IL, revisited a controversy that dates back nearly 15 years surrounding Medtronic’s Infuse bone graft, which contains a recombinant version of bone morphogenetic protein-2 (rhBMP-2). Women of childbearing age should be warned of potential risks to a fetus and should discuss other possible treatments with their doctor.įor more information about INFUSE Bone Graft please visit. Women of childbearing age should not become pregnant for one year following treatment with the product. This product has also not been studied in nursing mothers. This product has not been tested in pregnant women to determine if it could harm a developing fetus.INFUSE Bone Graft should not be used in the vicinity of a resected or extant tumor, in patients with any active malignancy or patients undergoing treatment for malignancy.INFUSE Bone Graft should not be used in patients with an active infection at the operative site.INFUSE Bone Graft has not been studied in patient who are skeletally immature (This is most likely due to the influx of the patient’s own cells and fluids into the treatment site. The potential for prolonged swelling may occur in some (but not all) patients.Important Information about INFUSE ® Bone Graft: Supported by over 20 years of research and clinical results, including 60 preclinical studies and 5 clinical trials.Provides proven, predictable bone formation by combining the bone-generating power of rhBMP-2 with a proven carrier, the absorbable collagen sponge.Regenerates 100% vital, vascular de novo bone with no residual graft material remaining to destabilize bone formation.Eliminates the need for a second bone harvest surgery.Winner of the 2008 Prix Galien USA Award for best Biotechnology agent This led to the isolation of bone morphogenetic proteins (BMPs), the only proteins known to induce new bone formation (osteoinduction).Ģ002: INFUSE Bone Graft received approval for use in anterior lumbar spine fusion with Medtronic titanium threaded interbody devices.Ģ004: INFUSE Bone Graft received approval for use in open tibial fractures with intramedullary (IM) nail fixation.Ģ007: INFUSE Bone Graft received approval for use in sinus augmentation and for localized ridge augmentation for defects associated with extraction sockets.
Urist discovers that demineralized bone matrix stimulates the formation of new bone tissue in lower-order animal muscle. The history of rhBMP-2 extends back decades, providing a wealth of research and studies to support its ability to induce new bone formation.ġ965: Dr. Important information about INFUSE ® Bone Graft

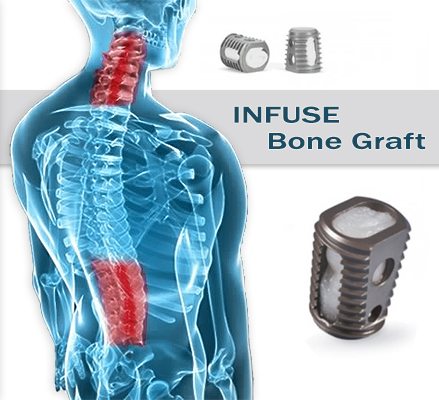
This particular protein has been proven to be an important element in the body’s own bone-healing cascade, inducing cells to form biologic bone via osteoinduction.

The active component of INFUSE Bone Graft - rhBMP-2- is a recombinantly-produced form of a signaling protein naturally occurring in the human body. INFUSE Bone Graft promotes natural bone growth to give you long-term implant success.


 0 kommentar(er)
0 kommentar(er)
